Abstract
BACKGROUND:. We have previously reported the first human application of non-viral gene transfer using the Sleeping Beauty (SB) transposon/transposase system to stably express a 2nd generation CD19-specific chimeric antigen receptor (CAR) which resulted in survival benefits for recipients of autologous and allogeneic T cells delivered as adjuvant immunotherapy after hematopoietic stem cell transplantation (HSCT) (J Clin Invest. 2016 126(9):3363-76). This CAR (CD19RCD28) used a Fc-based extracellular stalk and T cells were generated after 28 days (4 "stimulations") of recursive culture on activating and propagating cells (AaPC). To further improve persistence we modified this CAR construct and shortened the ex vivo time to produce clinical-grade T cells.
METHODS: A new Phase 1 clinical trial (NCT02529813) at MD Anderson Cancer Center enrollspatients with a history of CD19+ lymphoid malignancy with active disease for dose escalation of SB-modified autologous T cells which were infused following lymphodepletion chemotherapy, starting at ≤105 (dose level, DL -1), >105 but ≤106 (dose level, DL 1) and then escalating in cohorts of 3 to the dose of >108 but ≤109 CD3+CAR+ cells/kg (DL 4). The CD19-specific CAR employed a revised stalk derived from CD8, and as per CD19RCD28, signaled through chimeric CD28 and CD3-z endodomains (designated CD19RCD8CD28). SB-derived DNA plasmids coding for CAR (transposon) and transposase were electro-transferred into peripheral blood mononuclear cells and subsequently co-cultured every ~7 days on irradiated K-562-derived CD19+ AaPC in the presence of IL-2 and IL-21 to selectively propagate CAR+ T cells. During dose escalation, T-cell manufacturing time was shortened by reducing the number of additions of AaPC from four to two stimulations. The final product met release criteria and was thawed prior to infusion.
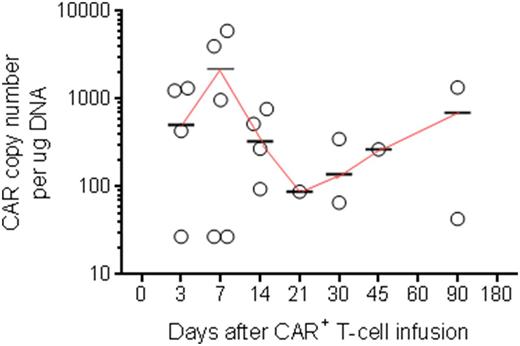
RESULTS: In this ongoing trial, 6 patients with median age 41 years (range 29-69 years) with active CD19+ lymphoid malignancy (CLL, n=1; ALL, n= 4; DLBCL, n=1) have been dosed so far (DL -1, n=1; DL 1, n=3; DL 2, n=2). All had refractory/relapsed disease with four having prior allogeneic HSCT. Median CAR expression upon infusion was 86% (range 19%-97%). No DLTs or CD19 related SAEs were observed. Only grade 1 cytokine release syndrome (CRS) was noted in 2 patients. The median follow-up for patients is 3 months (range 0.3-13.8 months). Anti-tumor effects were noted and infused T cells could be detected by flow cytometry and PCR (Figure).
CONCLUSIONS: These initial data describing the infusion of SB-modified T cells with a revised extracellular domain and under reduced manufacturing time results in the detection of genetically modified T cells and anti-tumor effects in patients with relapsed/refractory ALL apparently without clinically-significant CRS. These data support advancing an approach for non-viral gene transfer to administer CAR+ T cells, and working toward even shorter manufacturing times.
Figure: Persistence of circulating CD19-specific CAR+ T cells in patients after single infusion of SB-modified T cells, as determined by ddPCR. Circles represent a patient at given time point, short horizontal lines represent mean, and the continuous line connecting the means describes the trend.
Huls: Former employee of Intrexon: Employment. Smith: Ziopharm: Employment. De Groot: Ziopharm: Employment. Kantarjian: Amgen: Research Funding; ARIAD: Research Funding; Novartis: Research Funding; Pfizer: Research Funding; Bristol-Meyers Squibb: Research Funding; Delta-Fly Pharma: Research Funding. Cooper: Miltenyi Biotec: Honoraria; Ampliphi Biosciences: Equity Ownership; Procter & Gamble: Equity Ownership; Organovo Holdings: Equity Ownership; Targazyme, Inc.: Equity Ownership; Argos Therapeutics: Equity Ownership; Intrexon Corporation: Equity Ownership, Patents & Royalties; Immatics: Equity Ownership, Patents & Royalties, Research Funding; Sangamo Biosciences: Patents & Royalties; Ziopharm: Employment, Equity Ownership, Research Funding; Ferring: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal